Abstract
Background : Midostaurin (M) is an oral, multi-targeted, small molecule FLT3 inhibitor with single agent activity in both internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutant (mut) FLT3 AML. This global randomized, double-blinded placebo-controlled phase III trial demonstrated a statistically significant overall survival (OS) benefit when M was added to induction and consolidation chemotherapy followed by maintenance compared to standard chemotherapy in patients (pts) with activating FLT3 muts (HR=0.78, one sided p=0.0001; Stone et al NEJM, 2017). We now report a detailed comparison of CIR in each arm to determine if M significantly lowered the risk of relapse.
Methods : Between May 2008 and October 2011, 3277 previously untreated AML pts age 18-59 years in 17 countries were screened at one of 7 academic labs. 717 pts were randomized for the duration of therapy to M or placebo (P), stratified by FLT3 mut subtype (TKD v ITD high allelic mut fraction (>0.7) vs low mut fraction (0.05-0.7). Induction therapy consisted of daunorubicin 60 mg/m2 IV d1-3 and cytarabine (C) 200 mg/m2 day (d) 1-7 continuous intravenous infusion plus M or P (50 mg orally twice daily, d8-22). Re-treatment with a second blinded course was allowed if residual AML was noted on a d 21 marrow exam. Pts achieving complete remission (CR; < 5 % marrow blasts, ANC>1K/ul and platelets >100K/ul) received 4 cycles of C 3g/m2 over 3 h every 12 h d 1, 3, and 5 plus M or P (50 mg orally twice daily, d8-22) followed by twelve 28 day cycles of maintenance therapy with M or P (50 mg orally twice daily). Allogeneic transplantation (SCT) was allowed but not mandated; 409 pts underwent SCT. Treating death as a competing risk and stratifying on FLT3 subgroups, CIR analyses were performed using Gray's test (stratG) for two definitions of complete remission: CR per protocol (CR60; by day 60) and CR during induction (CRind; before protocol consolidation). CIR analyses treated relapses and AML deaths as events; deaths from other causes as competing risks; and survivors in CR as censored. A sensitivity analysis treated both transplants and non-AML deaths as competing risks to understand if M affected relapse early in the disease course.
Results : All pts are off active treatment, with a median follow-up of 59 months for surviving pts. Data for this analysis was frozen in April 2016. A CR was achieved within 60 days by 403 pts and 441 pts during induction with rates for M 'v' P: 59% v 54%, p=0.15; and 65% 'v' 58%, p=0.053, respectively). 181 CR60 and 199 CRind pts relapsed (M 'v' P: 43% v 53%, p=0.40; and 42% v 49%, p=0.15, respectively). As expected, a higher percentage of pts in this subset had normal or favorable modified ELN (based on cytogenetics and FLT3 mut status) compared to those not included in this analysis; however, pretreatment characteristics for these pts were balanced between the arms and were not significantly different from the 717 pts who were initially randomized (Table).
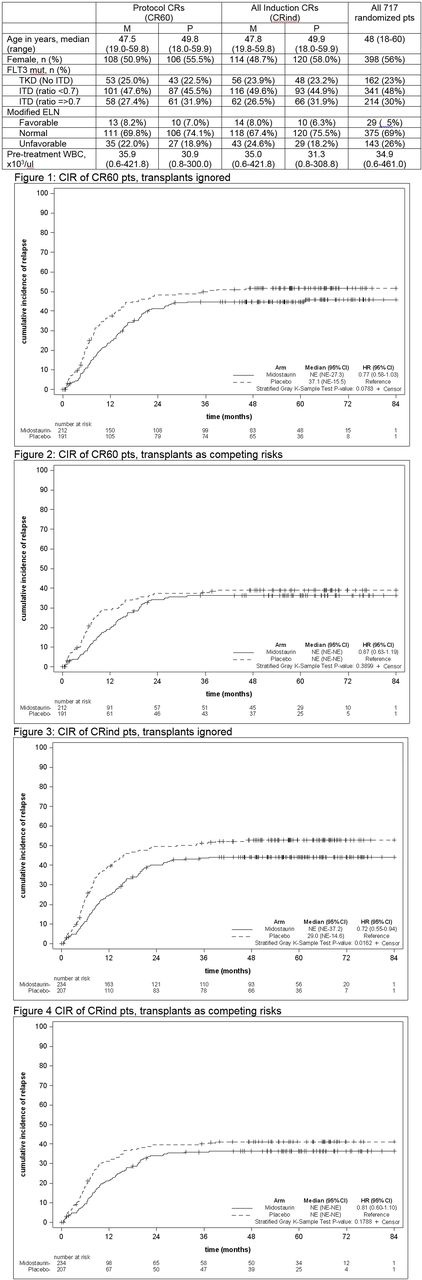
In both the CR60 and CRind pts, M had a lower CIR than P when SCT status was ignored (CR60: HR=0.77 [95% CI: 0.58-1.03], stratG p=0.08; CRind: 0.72 [0.55-0.94], stratG p=0.02) (see Figs 1 and 3). However, in sensitivity analyses including transplant as a competing risk, this effect was lost (CR60: 0.87 (0.63-1.19), stratG p=0.34; CRind: 0.81 (0.60-1.10), stratG p=0.18) (see Figs 2 and 4). 148 CR60 and 154 CRind pts underwent transplant in CR1; CRind pts who received M were less likely to relapse (CR60: HR=0.62 [0.33-1.14], stratG p=0.12; CRind: HR=0.47 [0.26-0.87], stratG p=0.02).
Conclusions : In a post-hoc analysis from the CALGB 10603 trial CIR was improved with M compared with P irrespective of the definition of CR in analyses where transplant was not accounted for; when transplant was treated as a competing risk there was not a meaningful difference between the treatment arms, suggesting that transplant in CR1 was also important in preventing relapse. Our observations, including the reported better survival for pts transplanted in CR1 for pts on the M compared to the P arm, suggest that midostaurin decreases the risk of relapse with no deleterious effect on survival, potentially by leading to a lower level of tumor burden and more lasting remissions.
Support: U10CA180821, U10CA180882, U10CA180791, U10CA180820, U10CA180888, U24CA196171, U10CA180863, and (CCSRI) 704970; Novartis Pharma;ClinicalTrials.gov Identifier: NCT00651261
Stone: Sumitomo: Consultancy; Astellas: Consultancy; Arog: Consultancy; Agios: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Ono: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Abbvie: Consultancy; Fuji Film: Consultancy; Pfizer: Consultancy. Thiede: Roche: Consultancy; Agendix: Employment; Bayer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Lo Coco: TEVA: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Lundbeck: Honoraria, Speakers Bureau. Wei: AbbVie, Celgene, Servier: Research Funding; AbbVie, Celgene, Novartis, Amgen, Servier: Honoraria; AbbVie, Celgene, Novartis, Amgen, Servier: Membership on an entity's Board of Directors or advisory committees. Dohner: Abbvie: Consultancy; Agios: Consultancy; Amgen: Consultancy; Celator: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Seattle Genetics: Consultancy; Sunesis: Consultancy. Larson: Amgen Inc.: Research Funding; Novartis: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal